Spinal Cord Injury News
A collection of posts on news, information and resources for those with spinal cord injuries.
Stem Cells From Patients’ Fat Can Help Treat Spinal Cord Injuries
A Mayo Clinic study shows stem cells derived from patients’ own fat are safe and may improve sensation and movement after traumatic spinal cord injuries. The findings from the phase 1 clinical trial appear in Nature Communications. The results of this early research offer insights on the potential of cell therapy for people living with spinal cord injuries and paralysis for whom options to improve function are extremely limited.
Read more of Stem Cells From Patients’ Fat Can Help Treat Spinal Cord Injuries »
Posted in Research for a Cure on April 2nd, 2024.
Chinese Brain Implant Restores Mobility in Paralyzed Patient
After three months of home-based rehabilitation training with the brain-machine interface, the patient was able to control a pneumatic glove through brain activity.
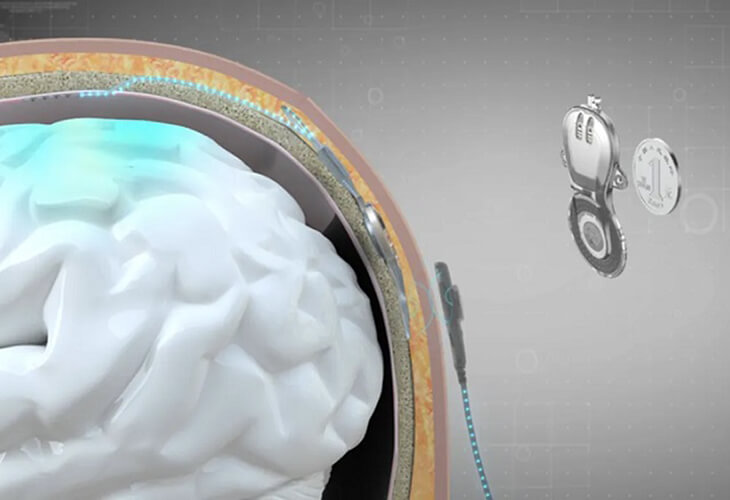
Just as Elon Musk’s Neuralink announced their success in putting an implant into a human brain, making headlines globally, China also showcases its latest progress in clinical application of brain-computer interfaces (BCIs), highlighting another emerging area of fierce competition between these 2 powers.
Read more of Chinese Brain Implant Restores Mobility in Paralyzed Patient »
Posted in Research for a Cure on February 1st, 2024.
Methods for Bypassing and Treating Spinal Cord Injury

Grégoire Courtine, Jocelyne Bloch and their research team have been breaking new ground in the treatment of neurological disorders for over a decade. Here’s a look at some of the promising new therapies they’ve developed.
Read more of Methods for Bypassing and Treating Spinal Cord Injury »
Posted in Research for a Cure on January 8th, 2024.
A Tiny Spinal Stimulator Could Someday Have a Big Impact on Paralysis
A device designed by Johns Hopkins researchers may hold promise for restoring mobility to those with lower limb paralysis.
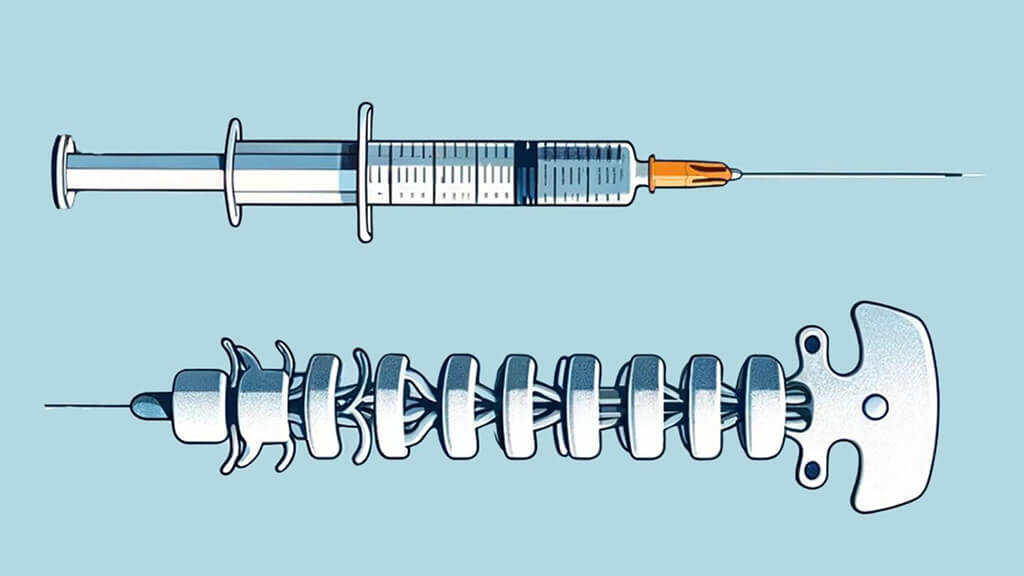
A Johns Hopkins materials scientist and collaborators have developed a tiny device that may hold promise for restoring mobility to those with lower limb paralysis, a condition affecting approximately 1.4 million Americans.
Read more of A Tiny Spinal Stimulator Could Someday Have a Big Impact on Paralysis »
Posted in Research for a Cure on November 27th, 2023.
Gene Therapy Developed to Restore Motor Function Across Complete Spinal Cord Injuries
Scientists at the NeuroRestore Centre in Switzerland have developed a gene therapy that was proven to stimulate nerve regrowth across complete spinal cord injuries in mice.
A complete spinal cord injury leads to irreversible paralysis. To combat this, the scientists’ gene therapy guides nerves to reconnect to their natural targets below the injuries in order to restore motor function.
Read more of Gene Therapy Developed to Restore Motor Function Across Complete Spinal Cord Injuries »
Posted in Research for a Cure on September 23rd, 2023.
